OUR CAPABILITIES

Containment
Containment and glovebox technology, including in box processes. We provide design solutions for active ventilation for high integrity environments.
READ MORE
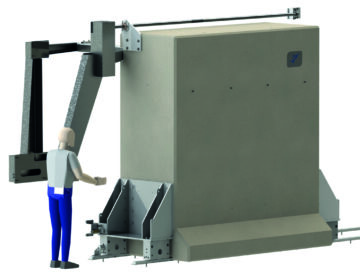
Shielded Facilities
Shielded facilities in concrete-steel or lead, including internal containment and processes. We provide solutions for all penetrations including shield doors, transfer tunnels and services.
READ MORE
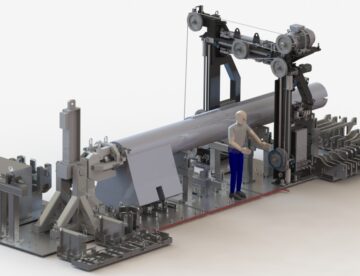
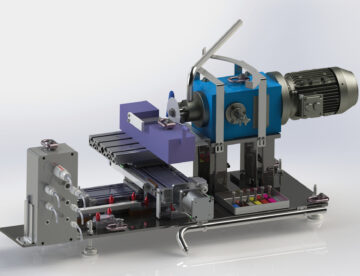
Remote Handling
Remote handling systems and processes for use inside shielded and contained facilities. We provide solutions for In Cell hoists, special In Cell remote machines and Master Slave manipulators.
READ MORE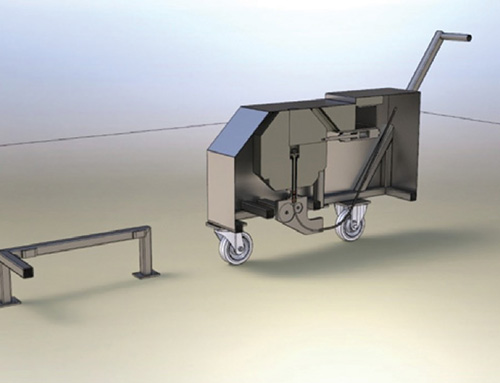
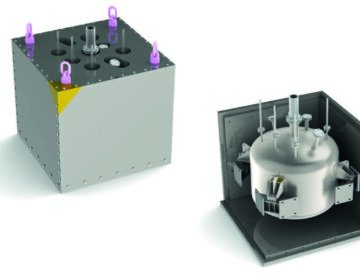
Transport & Packaging
Transport and packaging of product to and from the shielded and contained facilities, including flask bogies and rails, transport containers and posting systems.
READ MOREABOUT CYCLIFE AQUILA NUCLEAR
The CAN Team has an impressive track record in successfully delivering projects across a range of domestic and international nuclear market sectors.
Our vision is to be the company of choice, delivering mission critical nuclear engineering solutions in Europe, the Commonwealth and Pacific Rim. We combine proven management expertise with technical and process innovation to solve nuclear engineering challenges.
READ MOREACCREDITATIONS
FEATURE CASE STUDIES
 |
MFC BrochureFind out more about Aquila's Multi- Functional Cell. |
DOWNLOAD |
 |
Meet the teamMeet the leadership team at Aquila and discover their expertise. |
MEET THE TEAM |